Vapor pressure is caused by some molecules turning from a liquid into a gas.
(dibujo)
Pure Water
(dibujo)
solution of pre water and salt (not volatile)
Because there are now salt molecules taking up the spare surface, less molecules can turn to gas. So vapor pressure is lower.
knowing this we can assure that when there is more solute added to the solution, the vapor pressure will decrease.
Francois-Maire Raolt discovered that:
Vapor pressure of solution = vapor pressure of solvent x molar fraction of the solvent
(P) (Po) (Xo)
This is called Raolt's Law. It can only be used when we make a solution with a solute that is non-volatile (which means it will not turn to gas).
Problem:
At 35 ºC water has a vapor pressure of 3.2 kPa (finish)
viernes, 14 de marzo de 2014
viernes, 7 de marzo de 2014
Vapor Pressure and Intermolecular Forces
We are investigating vapor pressure to relate it to intermolecular forces. Vapor pressure is the pressure exerted by vapor in a thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The vapor pressure indicates a liquid's evaporation rate. it is realted to the tendency of particles to escape from the liquid (or solid). A substane that has a high vapor pressure at normal temperatures is referred to as volatile. Anne Marie Helmenstine, 2014)
Intermolecular forces are forces of attraction or repulsion which act between neighboring particles (atoms, molecules or ions).
 |
| Schlenk Tube: We put the substance in here and from the schlenk tube we can properly take out all the hair with the vaccum. |
 |
| Water Bath: We use this to heat up the sucstance to the desired temperature. |
 |
| Vaccum Line: We use this to take the air out of the schlenk tube. |
 |
| Vapor Pressure Sensor: We plug this into a laptop and our schlenk tube to record the vapor pressure of our substance. |
 |
| 2-Propanol or Isopropyl alcohol: chemical compound with the molecular formula C3H8O or C3H7OH. It's a colorless, flamavle hemical compound with a strogn odor. it has a melting point of 355.8 K and a molecular mass of 60.  |
 | ||||||||||
|
Table of chemical compounds showing their boiling points, vapor pressure and intermolecular forces.
Name of compound
|
Molecular formula
|
Diagram of structure
|
Boiling point (oC)
|
Temperatures when vapour pressure measured (oC)
|
Vapour pressure (kPa)
|
Types of intermolecular forces
|
Pentane
|
C5H10
|
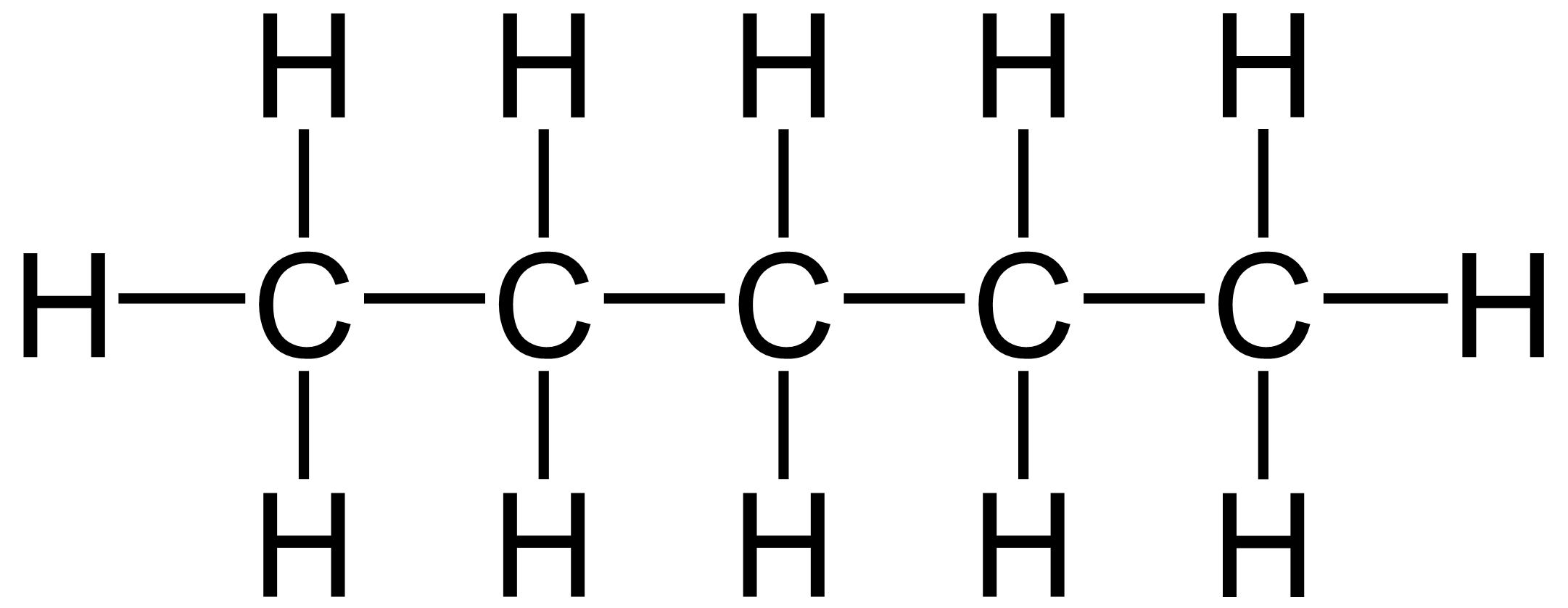 |
36
|
0
15
25
35
|
44.87
47
49.86
54
|
Van der Waal
|
Ethyl acetate
|
C4H8O2
|
 |
77,1
|
0
15
25
35
|
11.75
30.83
30.43
50.47
|
Van der Waal, permanent dipole dipole
|
Butyl acetate
|
C6H12O2
|
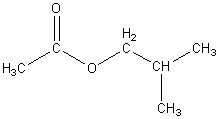 |
127
|
0
15
35
|
0,25
16,18
22,15
26,42
|
Van der Waal, permanent dipole dipole
|
1-Butanol
|
CH3(CH2)3
|
 |
117,7
|
0
15
25
35
|
0.63
1.46
2.72
3.66
|
Van der Waal, permanent dipole dipole, hydrogen
bonding
|
Propyl acetate
|
C5H10O2
|
101.6
|
0
15
25
35
|
9,1
20.66
20.89
21.54
|
Van der Waal, permanent dipole dipole
|
|
2-Propanol
|
C3H8O
|
 |
82
|
0
15
25
35
|
78.99
96.15
100.85
11.29
|
Van der Waal, permanent dipole dipole, hydrogen bonding
|
Explaination:
The boling point of pentane is the lowest one because of the intermolecular force it has. The only intermolecular force that pentane has is Van der Waal, so we can assume that the boiling point of this compound will be at a very low temperature.
The compound witht the highest boling point is butyl acetate, which doesn't have hydrogen bonding, that is the strongest type of bonding. Because of this reason we have to think that the permanent dipole dipole forces of this compound are really high.
1-Butanol and 2-Propanol have all 3 types of forces but their boling point is lower than butyl acetate. so the three forces of this compound must be waker than the two of butyl acetate.
References:
Anne Marie Helmenstine, P. 2014. Vapor Pressure Definition - Chemistry
 Glossary Definition of Vapor Pressure. [online] Available at: http://chemistry
Glossary Definition of Vapor Pressure. [online] Available at: http://chemistry .about.com/od/chemistryglossary/a/vaporpressdef.htm [Accessed: 7 Mar 2014].
.about.com/od/chemistryglossary/a/vaporpressdef.htm [Accessed: 7 Mar 2014].
Conservation of Mass
(Amrita Olabs, 2011)
As shown by our data, unlike mass volume
is not conserved. Especially in the case of gases, that don’t have a constant
volume (Lohninger H., 2014).
Molarity is the relationship between
the number of moles of solvent in a solution and the weight in Kg.
Molality is the relationship between
the number of moles of solvent in a solution and the volume in L.
References
Amrita Olabs.
2011. The Law of Conservation of Mass in a Chemical Reaction (Theory) :
Class 9 : Chemistry : Amrita Online Lab. [online] Available at:
http://amrita.olabs.co.in/?sub=73&brch=2&sim=118&cnt=1 [Accessed:
13 Mar 2014].
Lohninger, H.
2014. Volume Relationships in Chemical Reactions. [online]
Available at: http://www.vias.org/genchem/atommasses_12431_03.html [Accessed:
13 Mar 2014].
Nature.com.
2012. The Conservation of Mass | Learn Science at Scitable.
[online] Available at:
http://www.nature.com/scitable/knowledge/library/the-conservation-of-mass-17395478
[Accessed: 13 Mar 2014].
Suscribirse a:
Comentarios (Atom)



