Boyle-Marriote
Law:
Gas
Equation: Pi·Vi = Pf·Vf
Initial
Pressure(Pi) = Pf·Vf / Vi
Initial
Volume(Vi) = Pf·Vf / Pi
Final
Pressure(Pf) = Pi·Vi / Vf
Final
Volume(Vf) = Pi·Vi / Pf
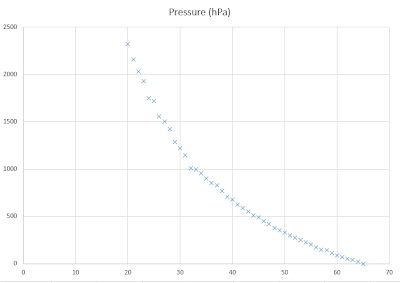
Conclusion: The lower the volume the higher the pressure. This is because when the same amount of particles are together in a big place there is plenty of space between them, but when they are all together in a smaller place the particles have a smaller space between them. When this happens the pressure is higher.

No hay comentarios:
Publicar un comentario